Solvent Solubility Chart
Solvent Solubility Chart - The solvent is the substance that dissolves the solute and the component of a chemical solution present in the greatest amount. Usually, a solvent is a liquid. A solvent can be solid, liquid or gas. A usually liquid substance capable of dissolving or dispersing one or more other substances. A solvent is a molecule that has the ability to dissolve other molecules, known as solutes. Solvents are often liquids, although they can also be solids, gases, or supercritical fluids. A solvent is a substance that dissolves a solute to form a homogeneous solution. Something that eliminates or attenuates something. A solvent is the component of a solution that is present in the greatest amount. A solvent is usually a liquid, but can also be a solid or gas. A solvent is the component of a solution that is present in the greatest amount. Solvents are often liquids, although they can also be solids, gases, or supercritical fluids. A usually liquid substance capable of dissolving or dispersing one or more other substances. Water is a solvent for polar molecules, and the most common solvent used by living things; A solvent is a substance that dissolves a solute to form a homogeneous solution. A solvent is a substance that becomes a solution by dissolving a solid, liquid, or gaseous solute. While most common solvent are liquids, a. Usually, a solvent is a liquid. The solvent is the substance that dissolves the solute and the component of a chemical solution present in the greatest amount. A solvent is a substance that can dissolve an insoluble solute and create a solution. Solvents are often liquids, although they can also be solids, gases, or supercritical fluids. A solvent is a substance that dissolves a solute to form a homogeneous solution. The molecules of the solvent work to put the. A solvent is a substance that becomes a solution by dissolving a solid, liquid, or gaseous solute. The solvent is the substance that. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Solvents are often liquids, although they can also be solids, gases, or supercritical fluids. A solvent is a substance that can dissolve an insoluble solute and create a solution. While most common solvent are liquids, a. Water is a solvent for polar. A solvent is a substance that dissolves a solute to form a homogeneous solution. A solvent is, by definition, any substance that will take other things (aka ‘solutes’) into solution. Polar solvents (e.g., water) favour formation of ion s; A solvent is a substance that can dissolve an insoluble solute and create a solution. While most common solvent are liquids,. A solvent is a substance that becomes a solution by dissolving a solid, liquid, or gaseous solute. The molecules of the solvent work to put the. A solvent is, by definition, any substance that will take other things (aka ‘solutes’) into solution. A solvent is a molecule that has the ability to dissolve other molecules, known as solutes. For example,. Essential traits include its dissolving power, homogeneity, and its role as a reaction medium. Solvent, substance, ordinarily a liquid, in which other materials dissolve to form a solution. Usually, a solvent is a liquid. Something that provides a solution. A solvent is the component of a solution that is present in the greatest amount. A solvent is a substance that can dissolve an insoluble solute and create a solution. A solvent can be solid, liquid or gas. A solvent is usually a liquid, but can also be a solid or gas. Solvents are often liquids, although they can also be solids, gases, or supercritical fluids. Polar solvents (e.g., water) favour formation of ion s; A solvent can be solid, liquid or gas. The molecules of the solvent work to put the. A solvent is a substance that becomes a solution by dissolving a solid, liquid, or gaseous solute. Something that eliminates or attenuates something. It is the substance in which the solute is dissolved. Something that eliminates or attenuates something. A solvent is, by definition, any substance that will take other things (aka ‘solutes’) into solution. The molecules of the solvent work to put the. Something that provides a solution. A solvent is the component of a solution that is present in the greatest amount. It is the substance in which the solute is dissolved. A solvent is, by definition, any substance that will take other things (aka ‘solutes’) into solution. A solvent is a substance that dissolves a solute to form a homogeneous solution. A solvent is a substance that becomes a solution by dissolving a solid, liquid, or gaseous solute. While most common. Solvents are often liquids, although they can also be solids, gases, or supercritical fluids. Water is a solvent for polar molecules, and the most common solvent used by living things; While most common solvent are liquids, a. A solvent is a substance that can dissolve an insoluble solute and create a solution. A solvent is a molecule that has the. The molecules of the solvent work to put the. A solvent is a substance that can dissolve an insoluble solute and create a solution. It is the substance in which the solute is dissolved. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. The solvent is the substance that dissolves the solute and the component of a chemical solution present in the greatest amount. A solvent is a molecule that has the ability to dissolve other molecules, known as solutes. A solvent is, by definition, any substance that will take other things (aka ‘solutes’) into solution. Water is a solvent for polar molecules, and the most common solvent used by living things; Something that provides a solution. Something that eliminates or attenuates something. Polar solvents (e.g., water) favour formation of ion s; A solvent is a substance that dissolves a solute to form a homogeneous solution. A solvent is a substance that becomes a solution by dissolving a solid, liquid, or gaseous solute. While most common solvent are liquids, a. A solvent is the component of a solution that is present in the greatest amount. For example, in a saltwater mixture, water functions as the solvent, and salt is the solute.Solvent Solubility Table
Solvent Solubility Chart
Solvent Solubility Chart
Solvent Solubility Chart
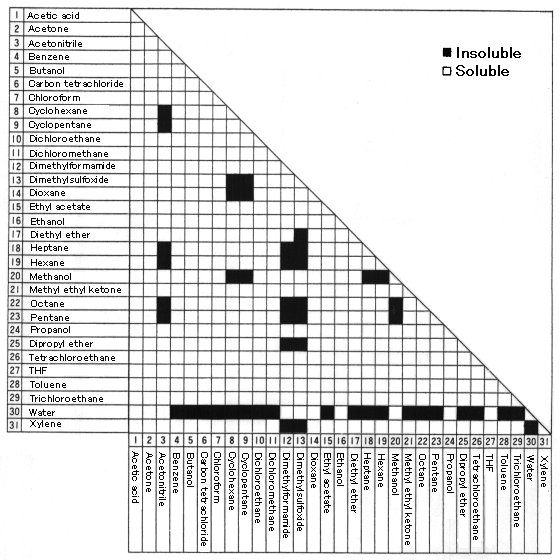
Organic_solvents Data With Water Solubility Solvent Functional Group
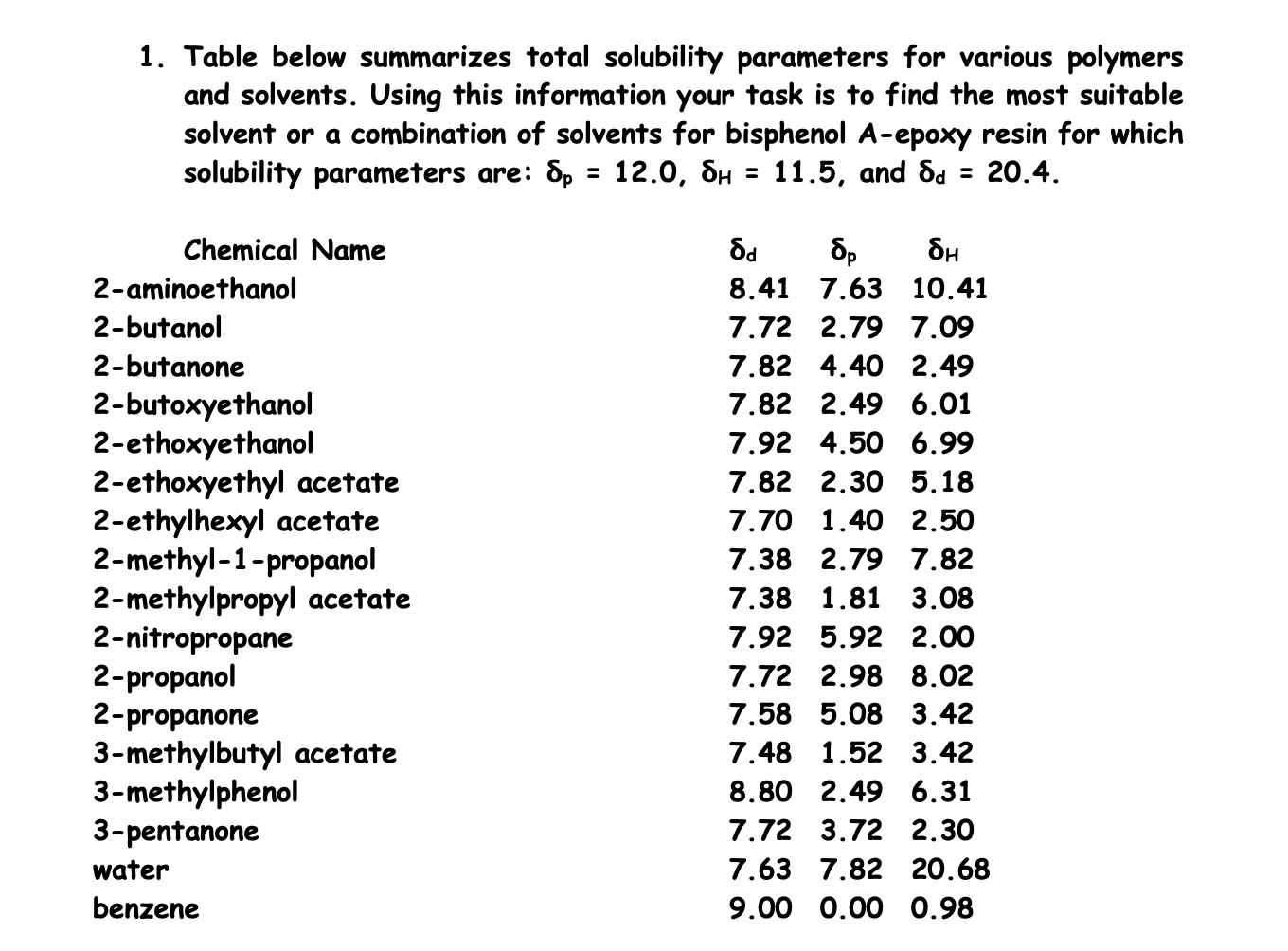
Solvent Solubility Chart
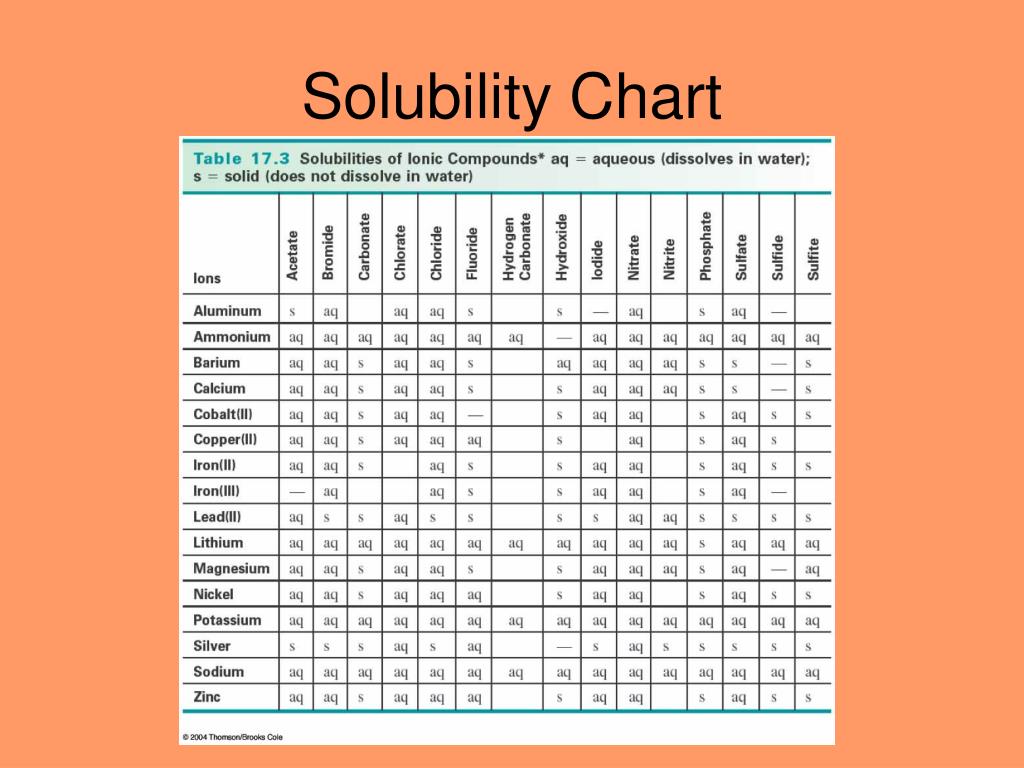
Solubility Rules Chart.png
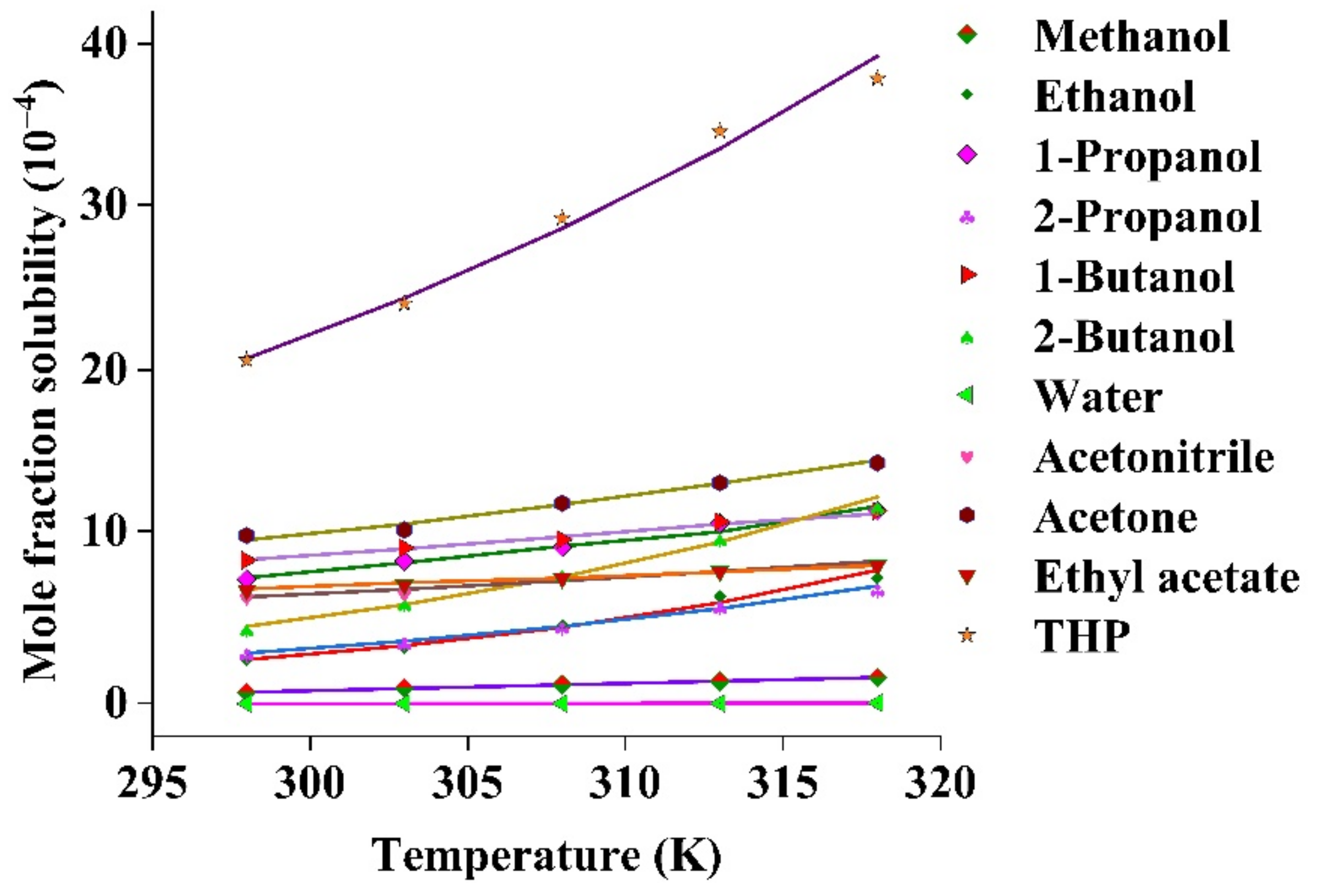
Common Organic Solvents_ Table of Properties Solvent Functional Group
Solubility Table
Solvent Solubility Table
Solvents Are Often Liquids, Although They Can Also Be Solids, Gases, Or Supercritical Fluids.
A Usually Liquid Substance Capable Of Dissolving Or Dispersing One Or More Other Substances.
Essential Traits Include Its Dissolving Power, Homogeneity, And Its Role As A Reaction Medium.
Usually, A Solvent Is A Liquid.
Related Post: