Oh Mary Seating Chart
Oh Mary Seating Chart - The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). The nitrate and the natrium ions. > basic oxides metallic character increases from right to left and from top to bottom in the periodic table. K sp = 5.5 × 10−11. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. Hydroxide anion, −oh, has a unit negative charge. Ignore the volume change associated with the added solid. Lithium is a group 1 metal and commonly forms a m + ion. Na_2co_3(aq) + 2agno_3(aq) rarr ag_2co_3(s)darr + 2nano_3(aq) the net ionic equation is: In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). The h (+) in the acid combines with the. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? A good leaving group has to be able to part with its electrons easily enough, so typically, it must be a strong acid or weak base relative to other substituents on the same. K sp = 5.5 × 10−11. When they make music together, there is thus 1:1 stoichiometry. Lithium is a group 1 metal and commonly forms a m + ion. > basic oxides metallic character increases from right to left and from top to bottom in the periodic table. Ignore the volume change associated with the added solid. Ignore the volume change associated with the added solid. > basic oxides metallic character increases from right to left and from top to bottom in the periodic table. K sp = 5.5 × 10−11. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. The h (+) in the acid combines with the. When they make music together, there is thus 1:1 stoichiometry. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt (neutralisation). In an aqueous solution containing 1.0 m nh4cl (k a = 5.56. Ulbb (standard reduction potentialscolor (white) (mmmmmll)e. The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). Ignore the volume change associated with the added solid. When an acid and a base are placed together, they react to. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. Lithium is a group 1 metal and commonly forms a m + ion. In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? The acid in excess is then titrated with n aoh (aq). Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. Hydroxide anion, −oh, has a unit negative charge. The h (+) in the acid combines with the. When they make music together, there is thus 1:1 stoichiometry. Na_2co_3(aq) + 2agno_3(aq) rarr ag_2co_3(s)darr + 2nano_3(aq) the net ionic equation is: K sp = 5.5 × 10−11. Ignore the volume change associated with the added solid. When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt (neutralisation). When they make music together, there is thus 1:1 stoichiometry. The nitrate and the natrium ions. In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. Hydroxide anion, −oh, has a unit negative charge. The h (+) in the acid combines with the. Lithium is a group 1 metal and. Ulbb (standard reduction potentialscolor (white) (mmmmmll)e. The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). Lithium is a group 1 metal and commonly forms a m + ion. The nitrate and the natrium ions. Now if. When they make music together, there is thus 1:1 stoichiometry. Hydroxide anion, −oh, has a unit negative charge. Ulbb (standard reduction potentialscolor (white) (mmmmmll)e. Ignore the volume change associated with the added solid. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. A good leaving group has to be able to part with its electrons easily enough, so typically, it must be a strong acid or weak base relative to other substituents on the same. The nitrate and the natrium ions. When they make music together, there is thus 1:1 stoichiometry. Lithium is a group 1 metal and commonly forms a m. The nitrate and the natrium ions. The h (+) in the acid combines with the. Hydroxide anion, −oh, has a unit negative charge. When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt (neutralisation). > basic oxides metallic character increases from right to left and from top to bottom in the periodic table. The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). K sp = 5.5 × 10−11. A good leaving group has to be able to part with its electrons easily enough, so typically, it must be a strong acid or weak base relative to other substituents on the same. When they make music together, there is thus 1:1 stoichiometry. Ignore the volume change associated with the added solid. Na_2co_3(aq) + 2agno_3(aq) rarr ag_2co_3(s)darr + 2nano_3(aq) the net ionic equation is: Ulbb (standard reduction potentialscolor (white) (mmmmmll)e.'Oh, Mary!' review — Cole Escola's wacky new comedy also has heart New York Theatre Guide
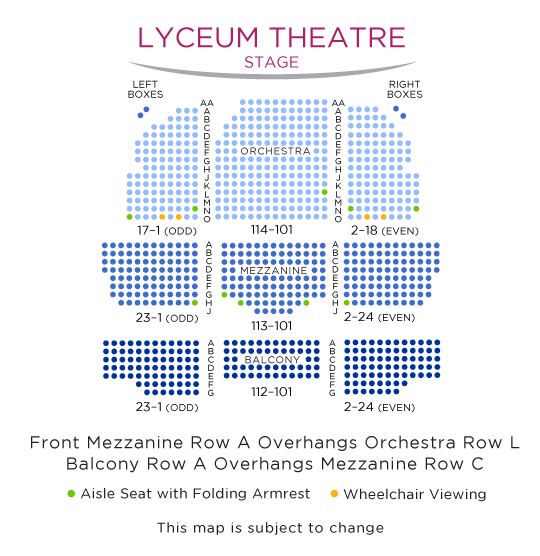
Lyceum Theatre Interactive Seating Chart Oh Mary!
Oh Mary Tickets Broadway Lyceum Theatre
Playhouse Square Mimi Theater at Iris Ingram blog
Lyceum Theater New York, NY Shows, Tickets, Seating Maps, Restaurants, Hotels, Parking and more
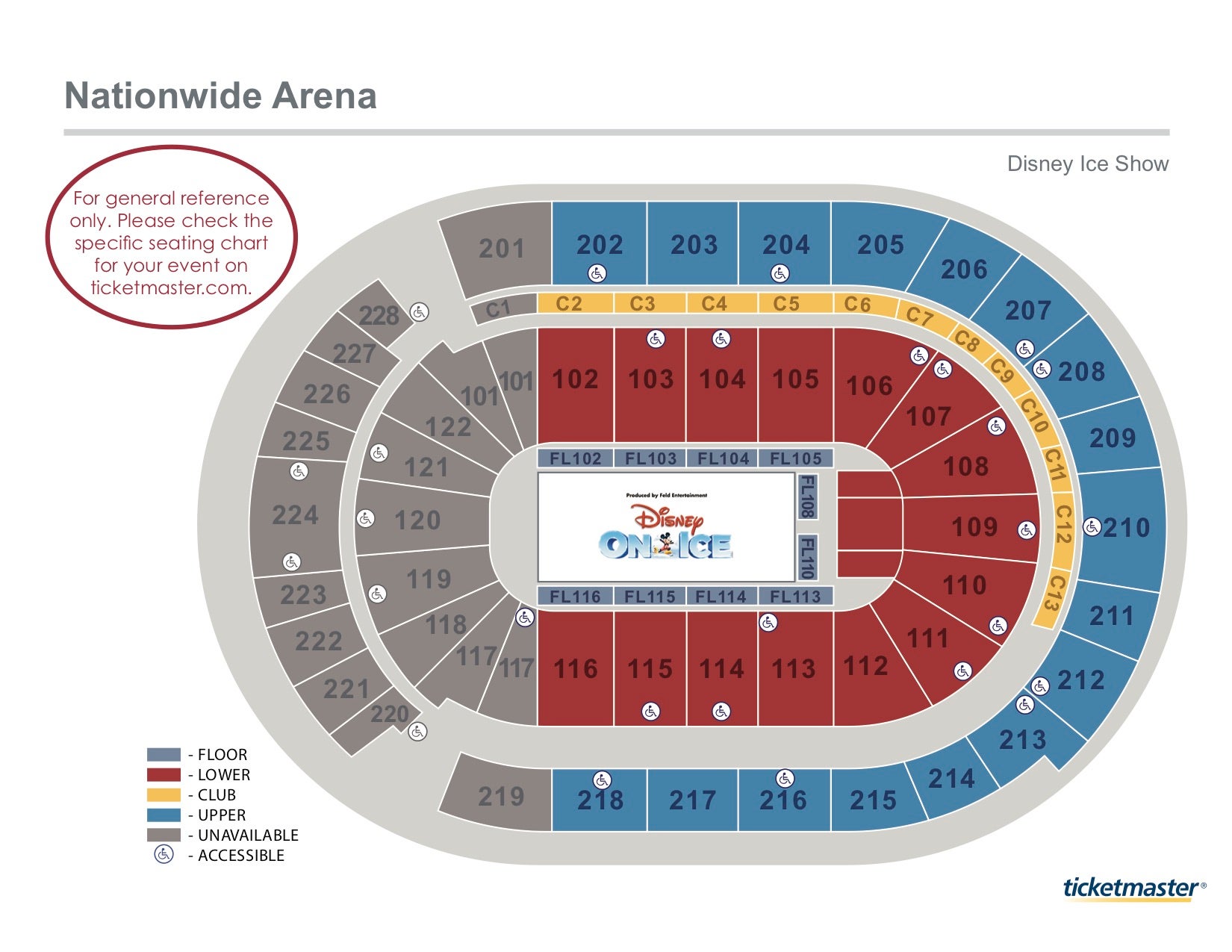
Blue Jackets Hockey Seating Chart Matttroy
The Realistic Joneses tickets access information, Broadway, New York, Play tickets
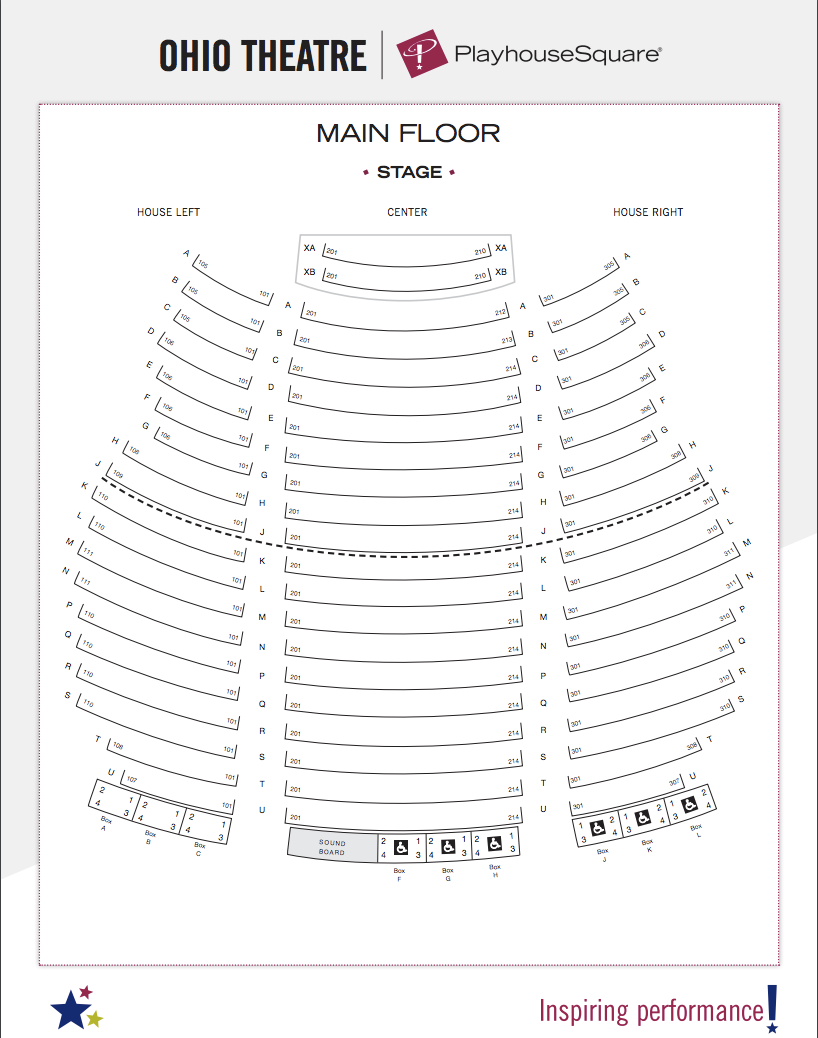
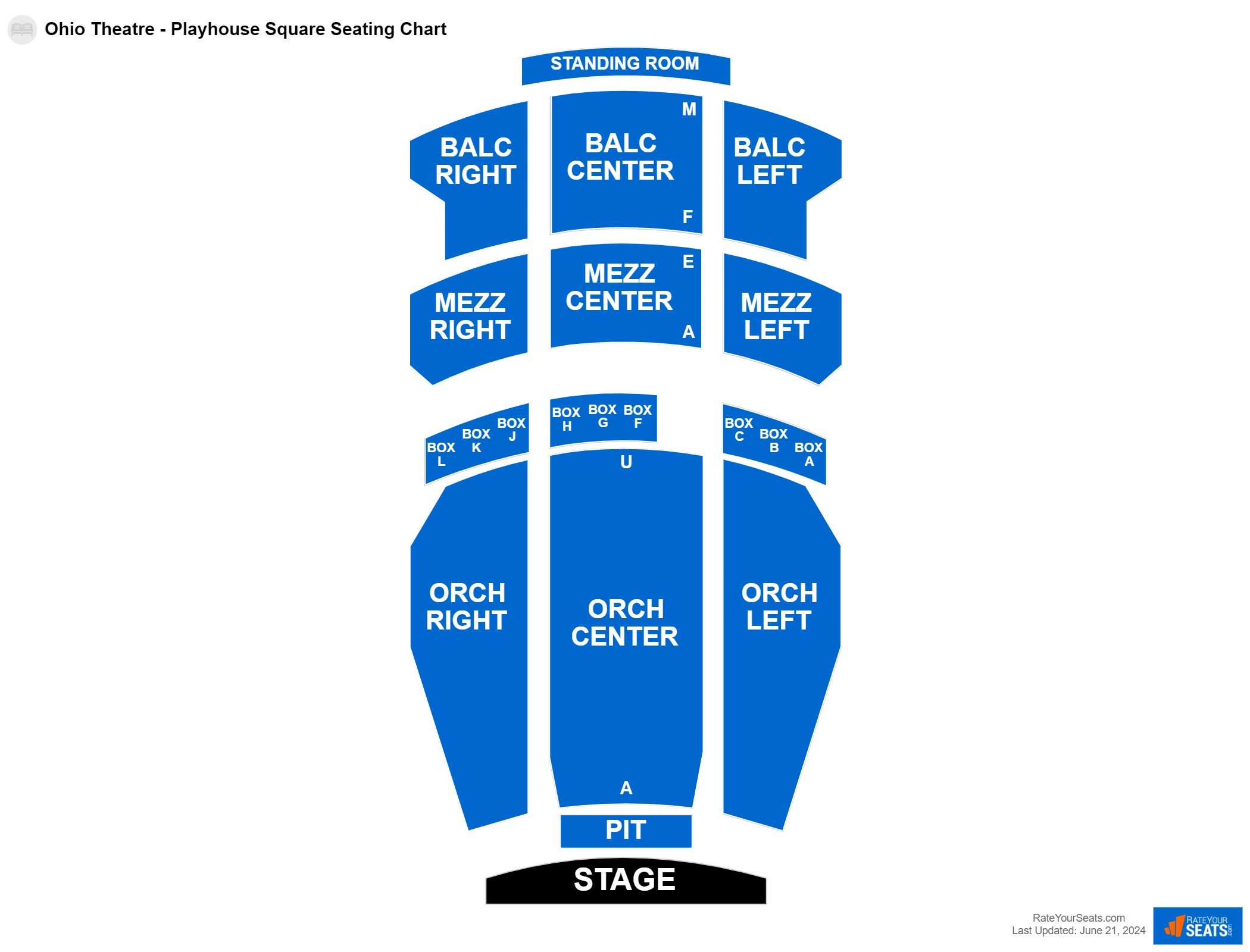
Playhouse Square Seating Chart
Cole Escola to star in new OffBroadway comedy 'Oh, Mary!' New York Theatre Guide
Mary Seating Chart Truly Inspired Paper Co.
In An Aqueous Solution Containing 1.0 M Nh4Cl (K A = 5.56 × 10−10), What Is The Solubility Of Mg(Oh)2?
Lithium Is A Group 1 Metal And Commonly Forms A M + Ion.
Now If The Parent Metal Has An Electronic Configuration Of 2:8:2, Then There Are 12 Electrons,.
Related Post: