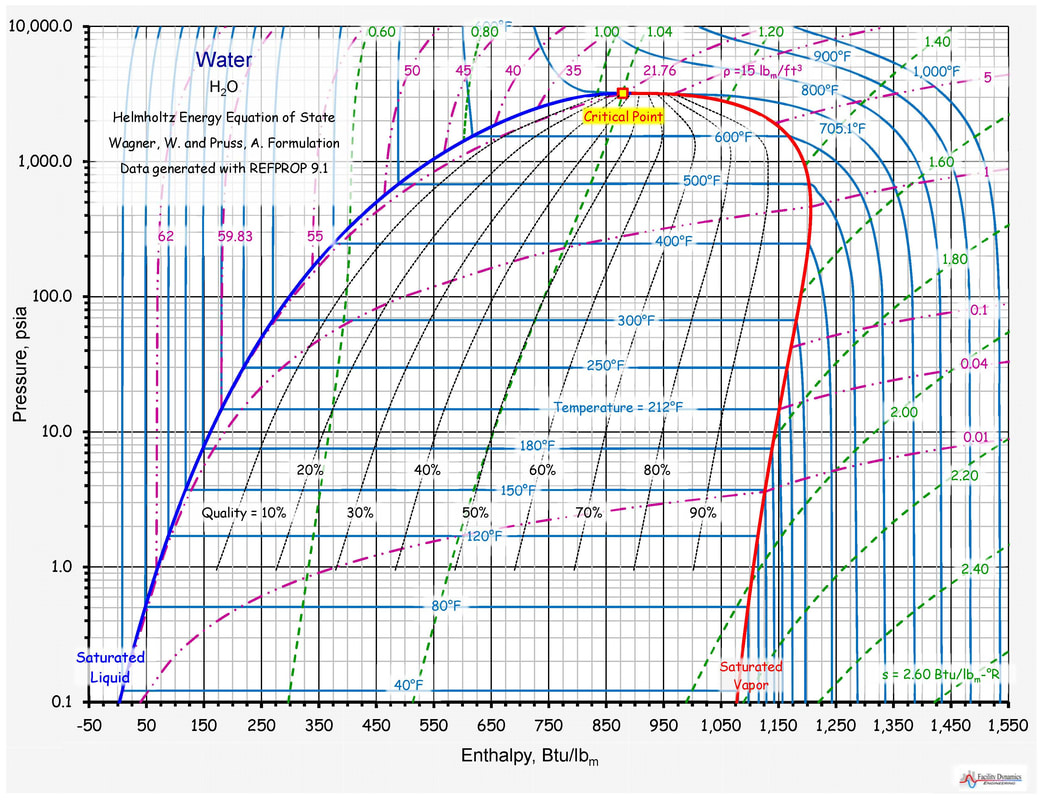
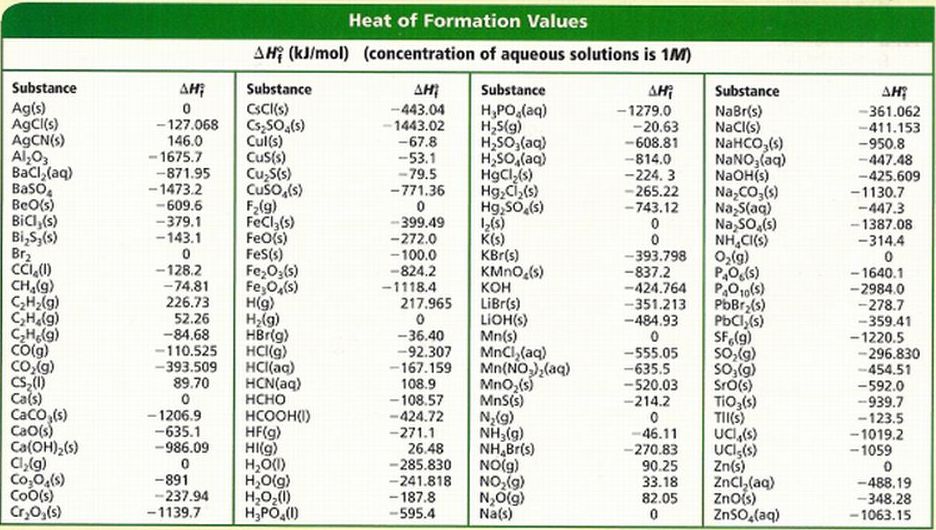
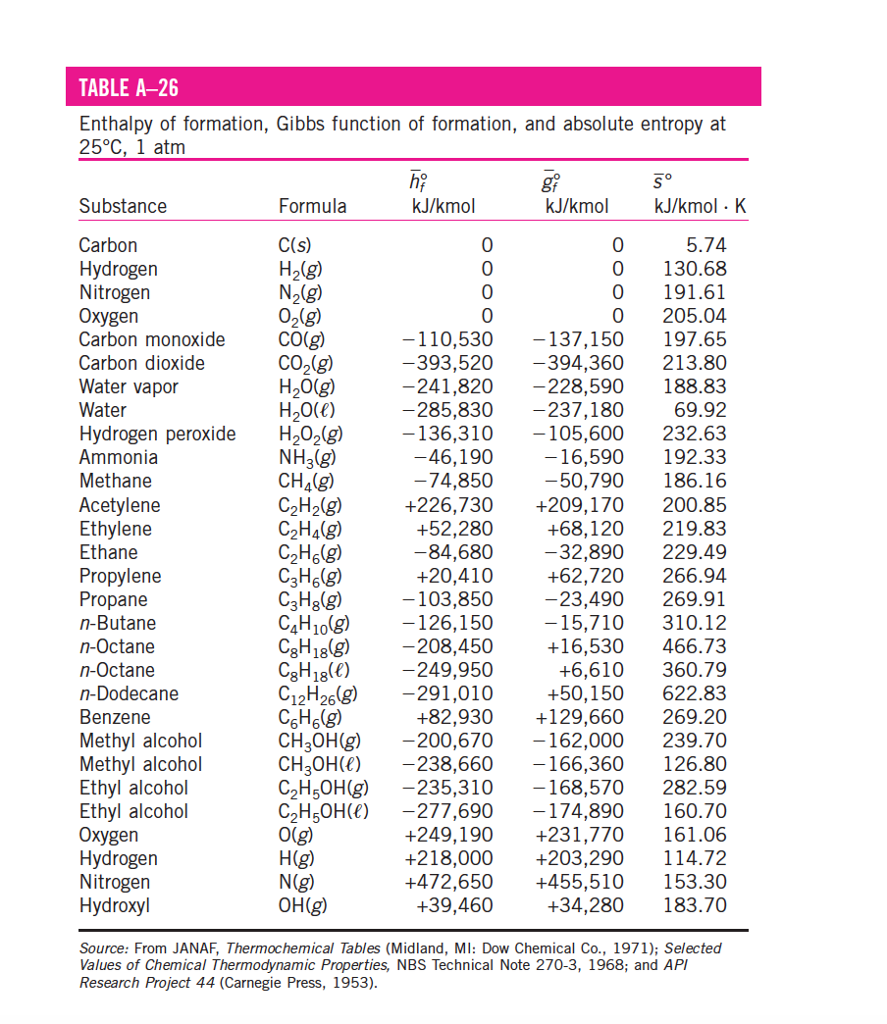
Enthalpy Chart
Enthalpy Chart - It is a property that helps scientists and engineers understand how. Check out a few examples and learn its formula. When a process occurs at. H = u + pv (1) (1) h = u + p v. What is enthalpy, and how to calculate it. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus. Enthalpy (/ ˈɛnθəlpi / ⓘ) is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many. In thermodynamics, the enthalpy is the measure of energy in a thermodynamic system. Enthalpy is the measurement of energy in a thermodynamic system. It is the thermodynamic quantity equivalent to the total heat content of a system. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: H = u + pv (1) (1) h = u + p v. When a process occurs at. Enthalpy (/ ˈɛnθəlpi / ⓘ) is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many. In thermodynamics, the enthalpy is the measure of energy in a thermodynamic system. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus. Check out a few examples and learn its formula. Enthalpy is the measurement of energy in a thermodynamic system. When a process occurs at. H = u + pv (1) (1) h = u + p v. In thermodynamics, the enthalpy is the measure of energy in a thermodynamic system. It is a state function in thermodynamics used in many. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume. Check out a few examples and learn its formula. It is a state function in thermodynamics used in many. Enthalpy is a fundamental concept in thermodynamics that governs the principles of energy conservation and transfer. When a process occurs at. What is enthalpy change, and how does temperature affect it. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: It is a state function in thermodynamics used in many. What is enthalpy. Enthalpy is the measurement of energy in a thermodynamic system. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus. It is the thermodynamic quantity equivalent to the total heat content of a system. It is a state function in thermodynamics used in many. Enthalpy (/ ˈɛnθəlpi / ⓘ). H = u + pv (1) (1) h = u + p v. Enthalpy (/ ˈɛnθəlpi / ⓘ) is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus. Check out a. H = u + pv (1) (1) h = u + p v. When a process occurs at. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus. What is enthalpy change, and how does temperature affect it. Check out a few examples and learn its formula. What is enthalpy change, and how does temperature affect it. When a process occurs at. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus. Enthalpy is the measurement of energy in a thermodynamic system. It is a state function in thermodynamics used in many. Enthalpy is the measurement of energy in a thermodynamic system. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: What is enthalpy, and how to calculate it. What is enthalpy change, and how does temperature affect it. It is a state function in. It is a state function in thermodynamics used in many. When a process occurs at. It is a property that helps scientists and engineers understand how. H = u + pv (1) (1) h = u + p v. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus. Enthalpy (/ ˈɛnθəlpi / ⓘ) is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. What is enthalpy, and how to calculate it. In thermodynamics, the enthalpy is the measure of energy in a thermodynamic system. When a process occurs at. It is a state function in thermodynamics used in many. It is the thermodynamic quantity equivalent to the total heat content of a system. The quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus. What is enthalpy change, and how does temperature affect it. Enthalpy is the measurement of energy in a thermodynamic system. It is a state function in thermodynamics used in many. H = u + pv (1) (1) h = u + p v. What is enthalpy, and how to calculate it. When a process occurs at. It is a property that helps scientists and engineers understand how. Enthalpy (/ ˈɛnθəlpi / ⓘ) is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. Check out a few examples and learn its formula.How To Read Pressure Enthalpy Diagram
Standard Enthalpy of Combustion MarielatinRobertson
Enthalpy Conversion Chart A Visual Reference of Charts Chart Master
40 How To Read Pressure Enthalpy Diagram Diagram For vrogue.co
Pressure Enthalpy Chart For Air
Air Enthalpy Chart at Elaine Paulson blog
Pressure enthalpy chart calculator jeryspecials
III Calculating Enthalpies STA Form IV Honors Chemistry Thermochemistry Unit
Nitrogen Enthalpy Chart
Enthalpy Chart Carrier Enterprise MidAtlantic Technical Support Site
In Thermodynamics, The Enthalpy Is The Measure Of Energy In A Thermodynamic System.
Enthalpy Is A Fundamental Concept In Thermodynamics That Governs The Principles Of Energy Conservation And Transfer.
Enthalpy (H H) Is The Sum Of The Internal Energy (U U) And The Product Of Pressure And Volume (Pv P V) Given By The Equation:
Related Post: