Dielectric Corrosion Chart
Dielectric Corrosion Chart - (few other solvents dissolve ions, polar aprotic almost never, exept ion pairs, but this is a different story) the dielectric constant. A dielectric with high permittivity ε ε permits (requires) more polarization for a given field magnitude than a low permittivity one. These dipoles will create a field that opposes the external field, resulting. The author chooses a surface such that the. This is higher than, say, glass. This is an example from the book. The dielectric is a very polar, protic solvent, presumably water. Do metals have an infinite permittivity? Bandgaps, as such, only exist in perfect crystals. Attach a voltage source (i.e., battery) to the capacitor. Because of this the value listed in a data sheet. I'm studying polarization, but i don't understand how i can solve the gauss's law for vector d. The dielectric is a very polar, protic solvent, presumably water. With no dielectric material (only vacuum) between the plates, the capacitor is actually easier to explain: Dielectric constant is a complex number. More polarization means more charge stored, so. Bandgaps, as such, only exist in perfect crystals. This is an example from the book. This is higher than, say, glass. It is a function of state variables, electric field, frequency, temperature, pressure, mechanical stress, etc. More polarization means more charge stored, so. A dielectric with high permittivity ε ε permits (requires) more polarization for a given field magnitude than a low permittivity one. Do metals have an infinite permittivity? Under the influence of an external electric field the dipoles in a dielectric medium arrange themselves. Attach a voltage source (i.e., battery) to the capacitor. With no dielectric material (only vacuum) between the plates, the capacitor is actually easier to explain: The author chooses a surface such that the. This is an example from the book. I'm studying polarization, but i don't understand how i can solve the gauss's law for vector d. Dielectric constant is a complex number. The dielectric is a very polar, protic solvent, presumably water. Dielectric constant is a complex number. A dielectric with high permittivity ε ε permits (requires) more polarization for a given field magnitude than a low permittivity one. Bandgaps, as such, only exist in perfect crystals. Do metals have an infinite permittivity? A dielectric with high permittivity ε ε permits (requires) more polarization for a given field magnitude than a low permittivity one. This is higher than, say, glass. Under the influence of an external electric field the dipoles in a dielectric medium arrange themselves. This is an example from the book. These dipoles will create a field that opposes the external. (few other solvents dissolve ions, polar aprotic almost never, exept ion pairs, but this is a different story) the dielectric constant. The author chooses a surface such that the. It is a function of state variables, electric field, frequency, temperature, pressure, mechanical stress, etc. With no dielectric material (only vacuum) between the plates, the capacitor is actually easier to explain:. Because of this the value listed in a data sheet. The dielectric is a very polar, protic solvent, presumably water. Bandgaps, as such, only exist in perfect crystals. Under the influence of an external electric field the dipoles in a dielectric medium arrange themselves. It is a function of state variables, electric field, frequency, temperature, pressure, mechanical stress, etc. Do metals have an infinite permittivity? It is a function of state variables, electric field, frequency, temperature, pressure, mechanical stress, etc. The dielectric is a very polar, protic solvent, presumably water. Dielectric materials tend to be more insulating than air, and thus by using such a material the plates (in a parallel plate capacitor) can be placed closer together which. Do metals have an infinite permittivity? Dielectric constant is a complex number. This is an example from the book. Under the influence of an external electric field the dipoles in a dielectric medium arrange themselves. With no dielectric material (only vacuum) between the plates, the capacitor is actually easier to explain: With no dielectric material (only vacuum) between the plates, the capacitor is actually easier to explain: Dielectric materials tend to be more insulating than air, and thus by using such a material the plates (in a parallel plate capacitor) can be placed closer together which would. A dielectric with high permittivity ε ε permits (requires) more polarization for a given. I'm studying polarization, but i don't understand how i can solve the gauss's law for vector d. Dielectric constant is a complex number. It is a function of state variables, electric field, frequency, temperature, pressure, mechanical stress, etc. These dipoles will create a field that opposes the external field, resulting. Under the influence of an external electric field the dipoles. Dielectric constant is a complex number. A dielectric with high permittivity ε ε permits (requires) more polarization for a given field magnitude than a low permittivity one. Do metals have an infinite permittivity? It is a function of state variables, electric field, frequency, temperature, pressure, mechanical stress, etc. (few other solvents dissolve ions, polar aprotic almost never, exept ion pairs, but this is a different story) the dielectric constant. More polarization means more charge stored, so. Because of this the value listed in a data sheet. The dielectric is a very polar, protic solvent, presumably water. Bandgaps, as such, only exist in perfect crystals. This is an example from the book. This is higher than, say, glass. Dielectric materials tend to be more insulating than air, and thus by using such a material the plates (in a parallel plate capacitor) can be placed closer together which would. With no dielectric material (only vacuum) between the plates, the capacitor is actually easier to explain: I'm studying polarization, but i don't understand how i can solve the gauss's law for vector d.Inconel Galvanic Corrosion Chart
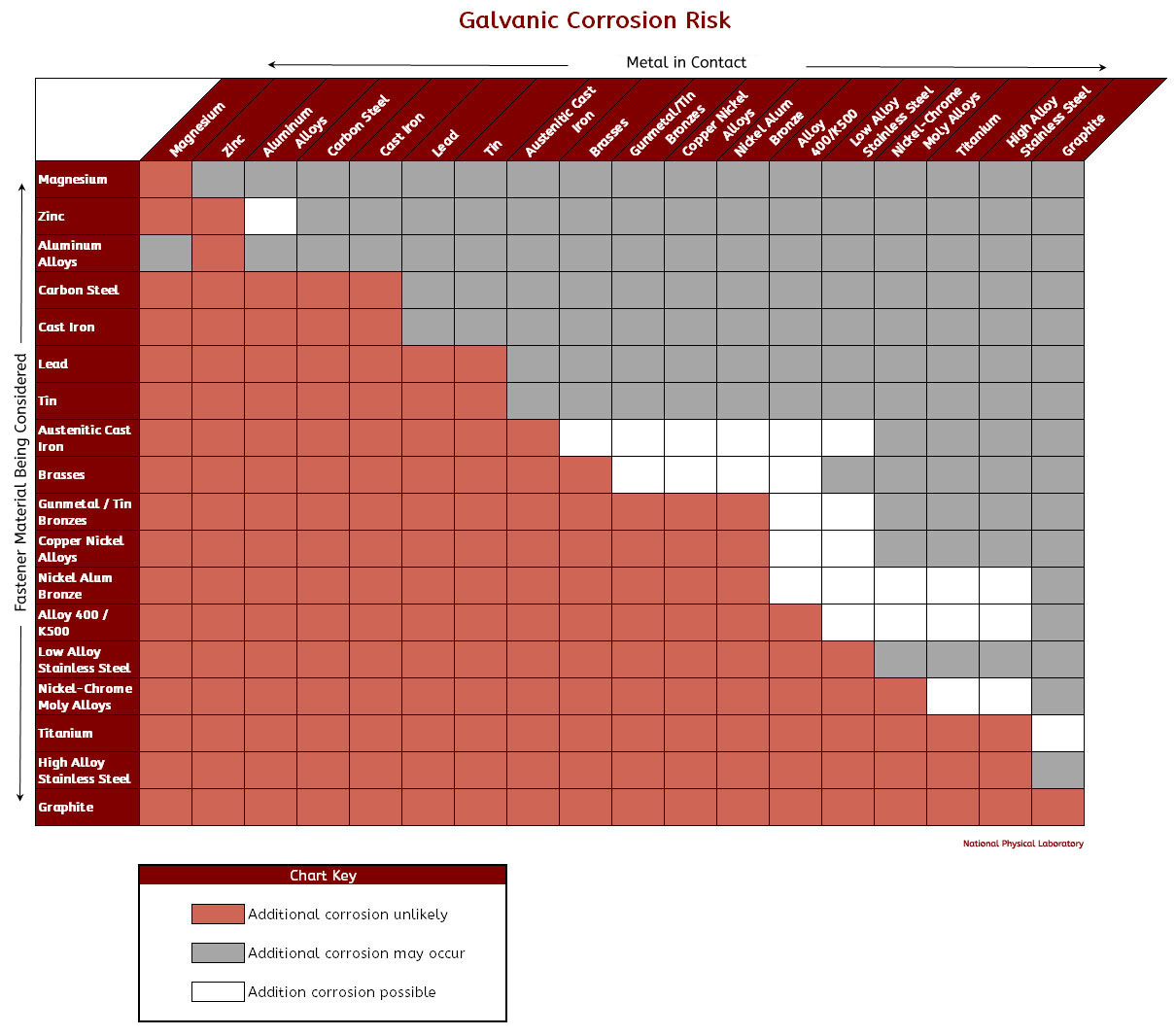
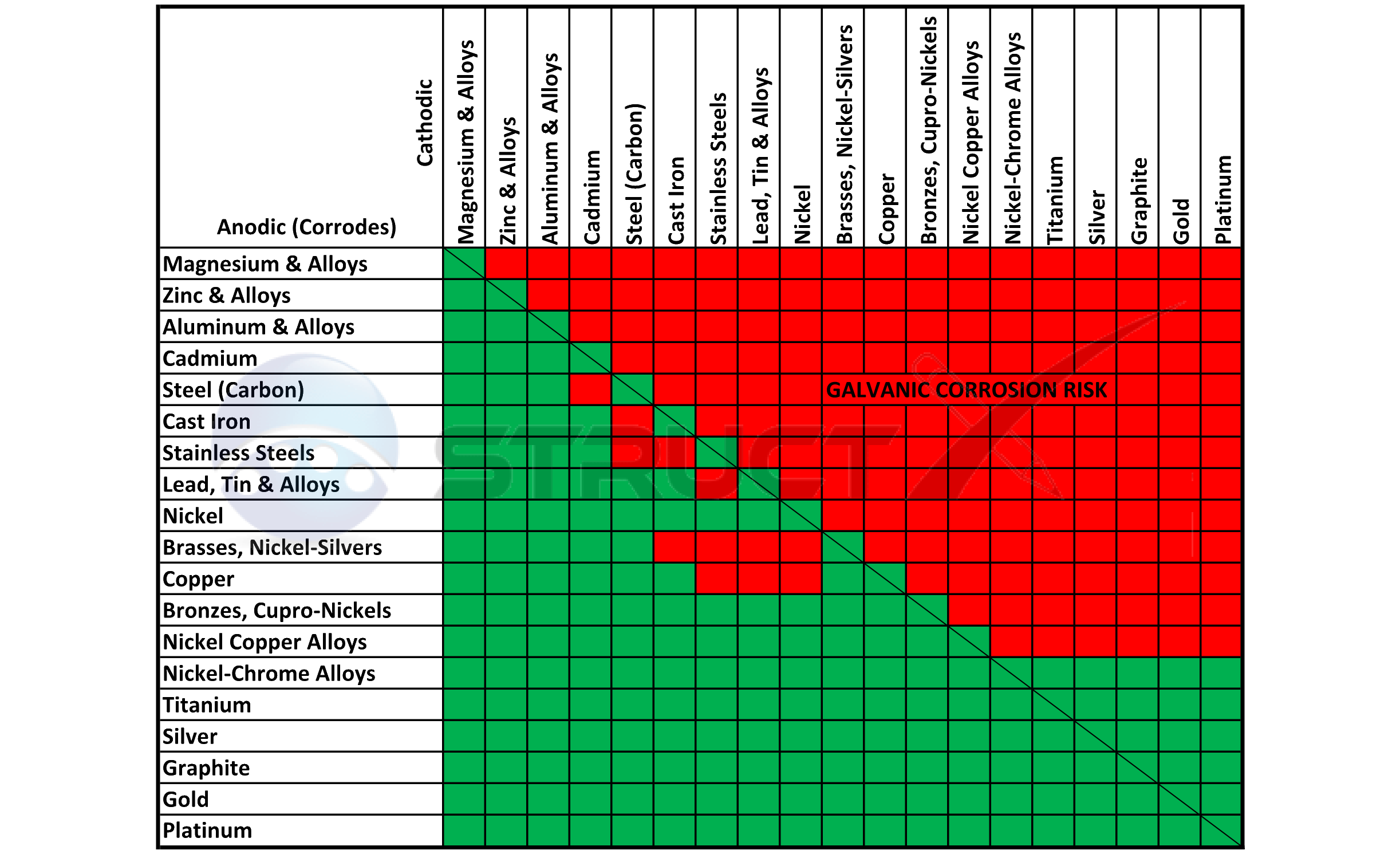
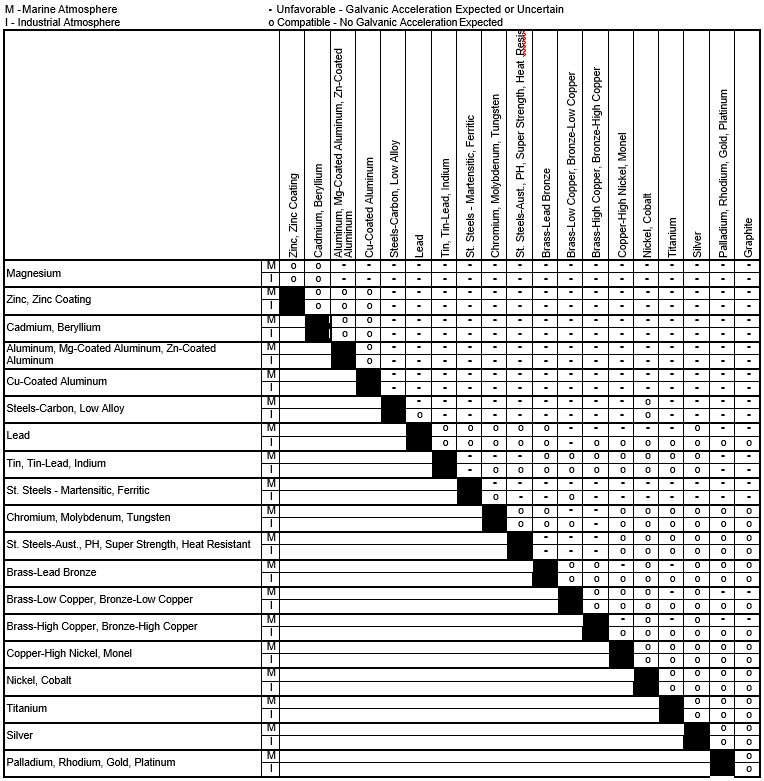
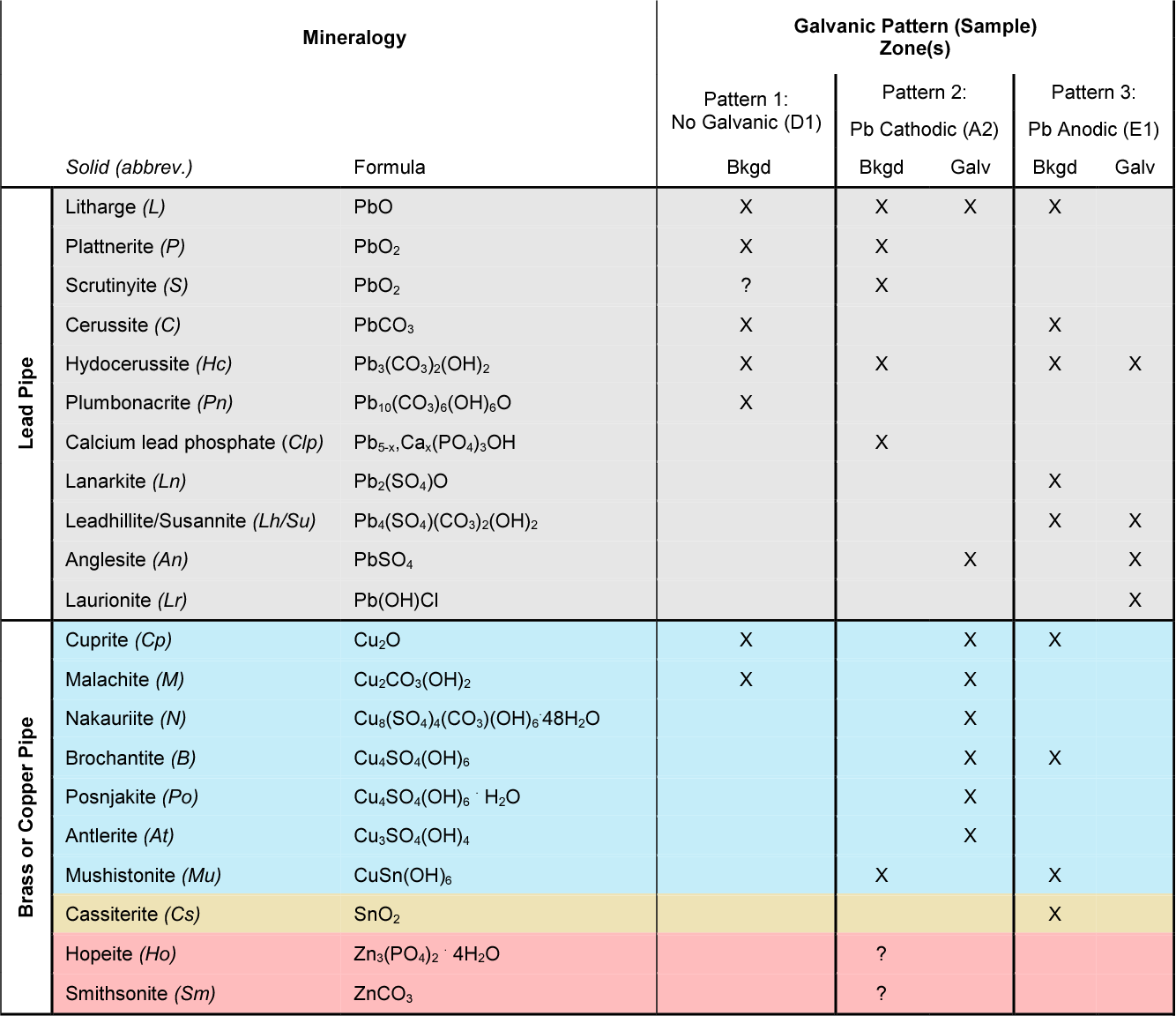
Galvanic Corrosion Chart
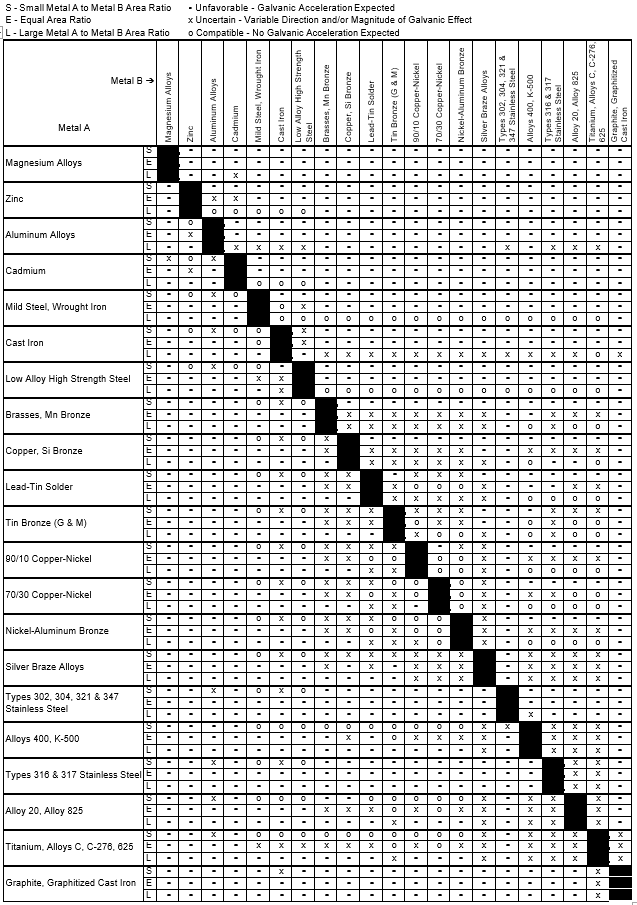
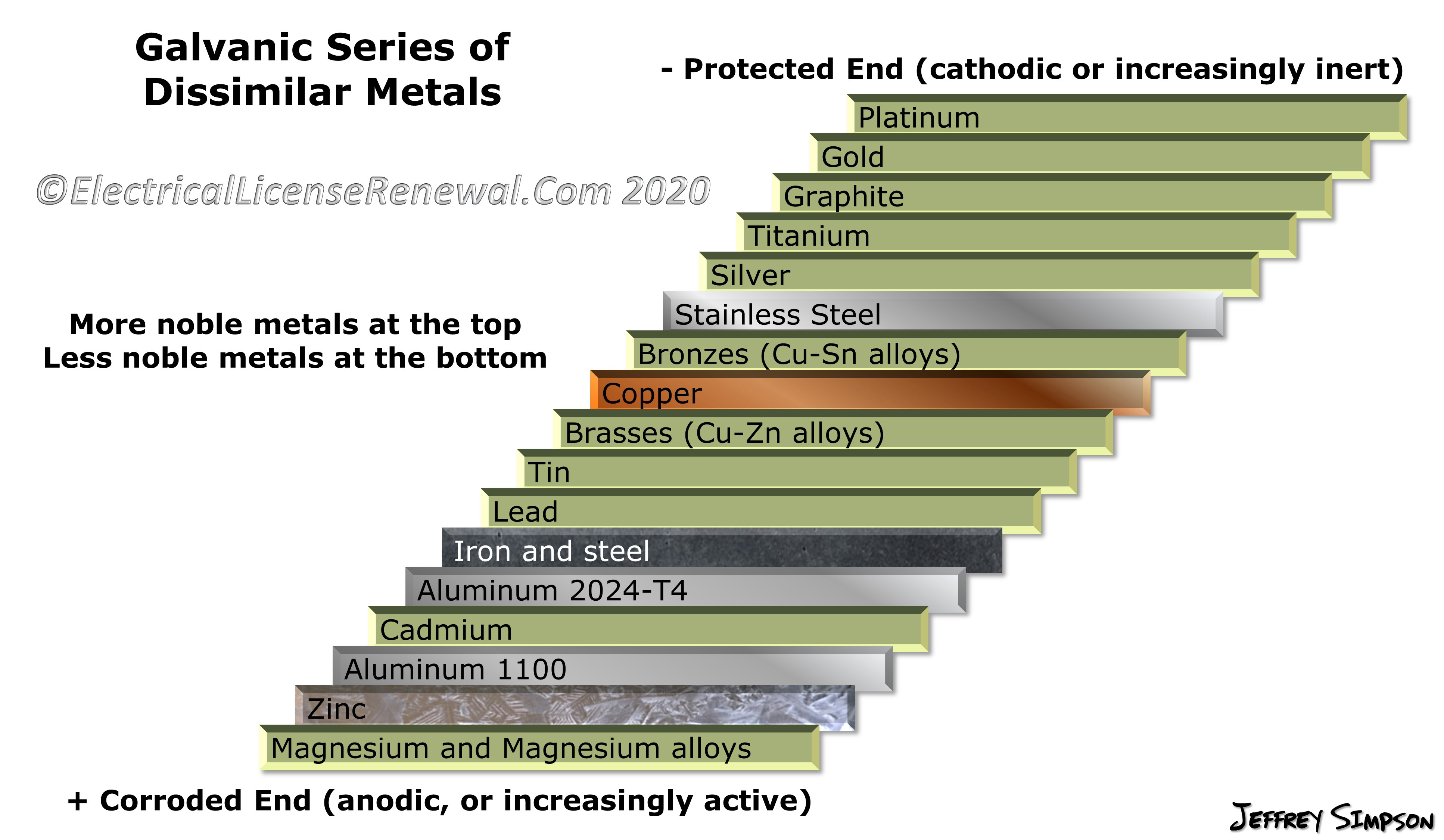
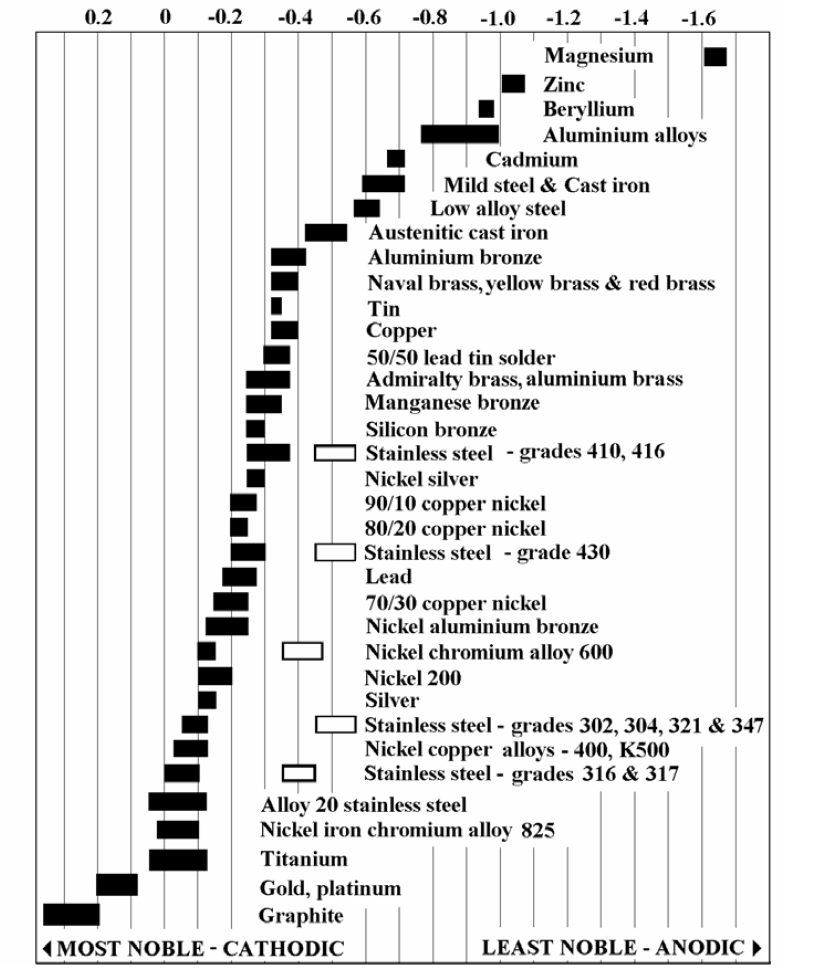
Galvanic Series (electrochemical series)
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Galvanic Action Chart
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Galvanic Corrosion SSINA
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Galvanic Series
Attach A Voltage Source (I.e., Battery) To The Capacitor.
The Author Chooses A Surface Such That The.
Under The Influence Of An External Electric Field The Dipoles In A Dielectric Medium Arrange Themselves.
These Dipoles Will Create A Field That Opposes The External Field, Resulting.
Related Post: