Amendment Chart
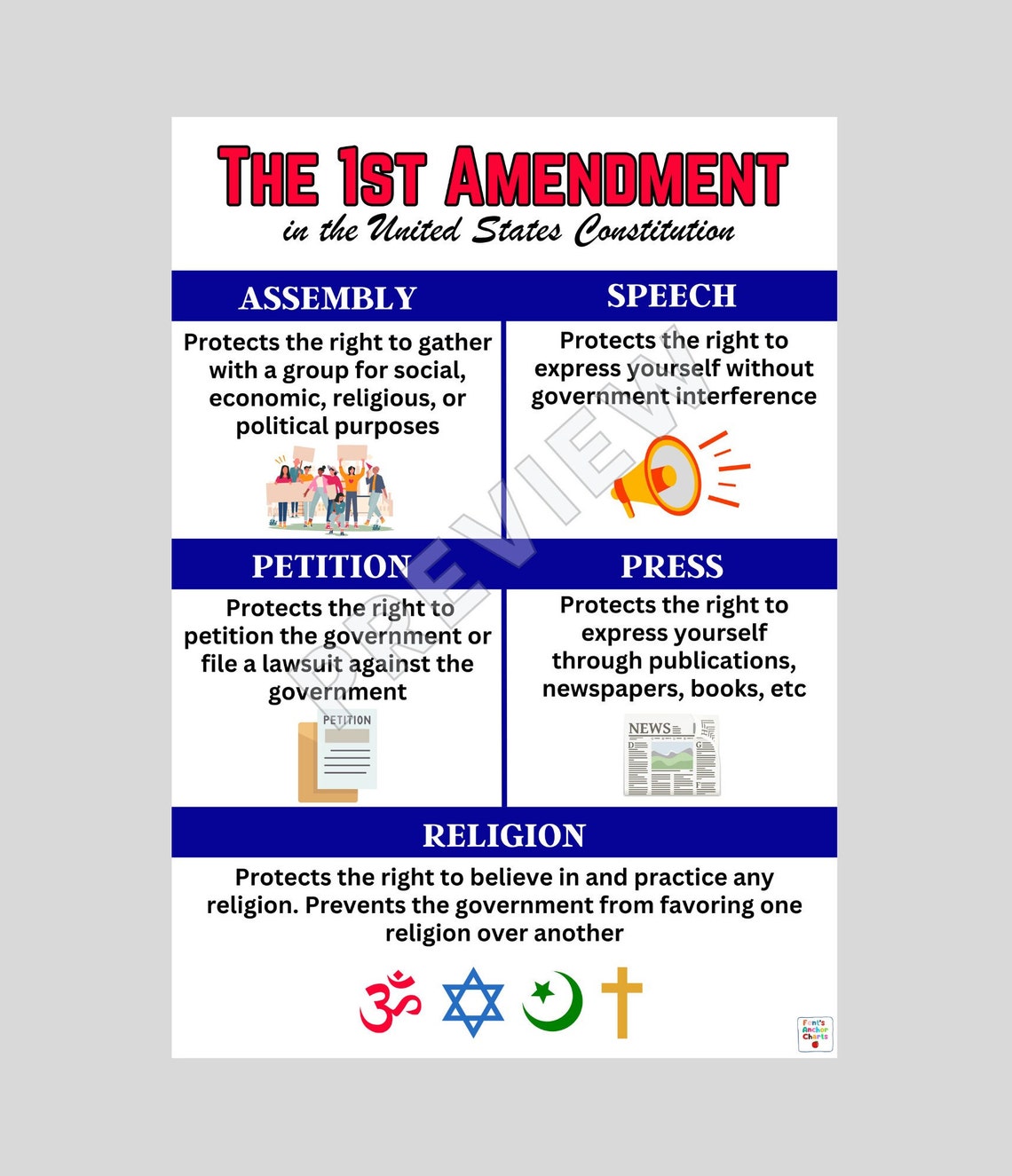
Amendment Chart - The irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111. Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. Investigators with an approved protocol must submit an amendment/modification of research form if there are significant changes involving any of the study protocols, study design,. The guidance put forth by byu human research protection program borrows extensively from the best practices of a variety of academic institutions. The information provided here builds on. Yes, if your study is currently approved through the pdf forms, you can continue to submit amendments on the pdf forms until you convert. To make changes to your approved study, please follow the instructions below. Amendments may be submitted at anytime, however, the change must not be. Overview you can only request modifications if your study is active and approved by the irb. Can i submit an amendment to my current irb? Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. Overview you can only request modifications if your study is active and approved by the irb. The information provided here builds on. The guidance put forth by byu human research protection program borrows extensively from the best practices of a variety of academic institutions. Investigators with an approved protocol must submit an amendment/modification of research form if there are significant changes involving any of the study protocols, study design,. Amendments may be submitted at anytime, however, the change must not be. To make changes to your approved study, please follow the instructions below. Yes, if your study is currently approved through the pdf forms, you can continue to submit amendments on the pdf forms until you convert. Can i submit an amendment to my current irb? The irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111. To make changes to your approved study, please follow the instructions below. Can i submit an amendment to my current irb? Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. Yes, if your study is currently approved through the pdf forms, you can continue to submit amendments on the pdf forms. Investigators with an approved protocol must submit an amendment/modification of research form if there are significant changes involving any of the study protocols, study design,. Overview you can only request modifications if your study is active and approved by the irb. Yes, if your study is currently approved through the pdf forms, you can continue to submit amendments on the. The irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111. The information provided here builds on. Yes, if your study is currently approved through the pdf forms, you can continue to submit amendments on the pdf forms until you convert. Before changes can be made. Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. The information provided here builds on. The guidance put forth by byu human research protection program borrows extensively from the best practices of a variety of academic institutions. Investigators with an approved protocol must submit an amendment/modification of research form if there. Investigators with an approved protocol must submit an amendment/modification of research form if there are significant changes involving any of the study protocols, study design,. Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. Can i submit an amendment to my current irb? To make changes to your approved study, please. Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. Amendments may be submitted at anytime, however, the change must not be. The irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111. Can i submit an amendment. The irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111. Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. To make changes to your approved study, please follow the instructions below. Amendments may be submitted at. Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. To make changes to your approved study, please follow the instructions below. The irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111. Overview you can only request. The irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111. The information provided here builds on. Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. Can i submit an amendment to my current irb? Overview you. Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. Overview you can only request modifications if your study is active and approved by the irb. The irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111. The. Can i submit an amendment to my current irb? Investigators with an approved protocol must submit an amendment/modification of research form if there are significant changes involving any of the study protocols, study design,. Yes, if your study is currently approved through the pdf forms, you can continue to submit amendments on the pdf forms until you convert. To make changes to your approved study, please follow the instructions below. Overview you can only request modifications if your study is active and approved by the irb. The information provided here builds on. Before changes can be made to an approved irb protocol, an amendment must be submitted to the irb. The irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111.First Amendment Anchor Chart, Bill of Rights Anchor Chart, Amendments Poster, United States
List 97+ Pictures Pictures Of The Constitution Completed
Amendments South Carolina Public Charter School District
PPT The Constitution & Federalism PowerPoint Presentation, free download ID4690649
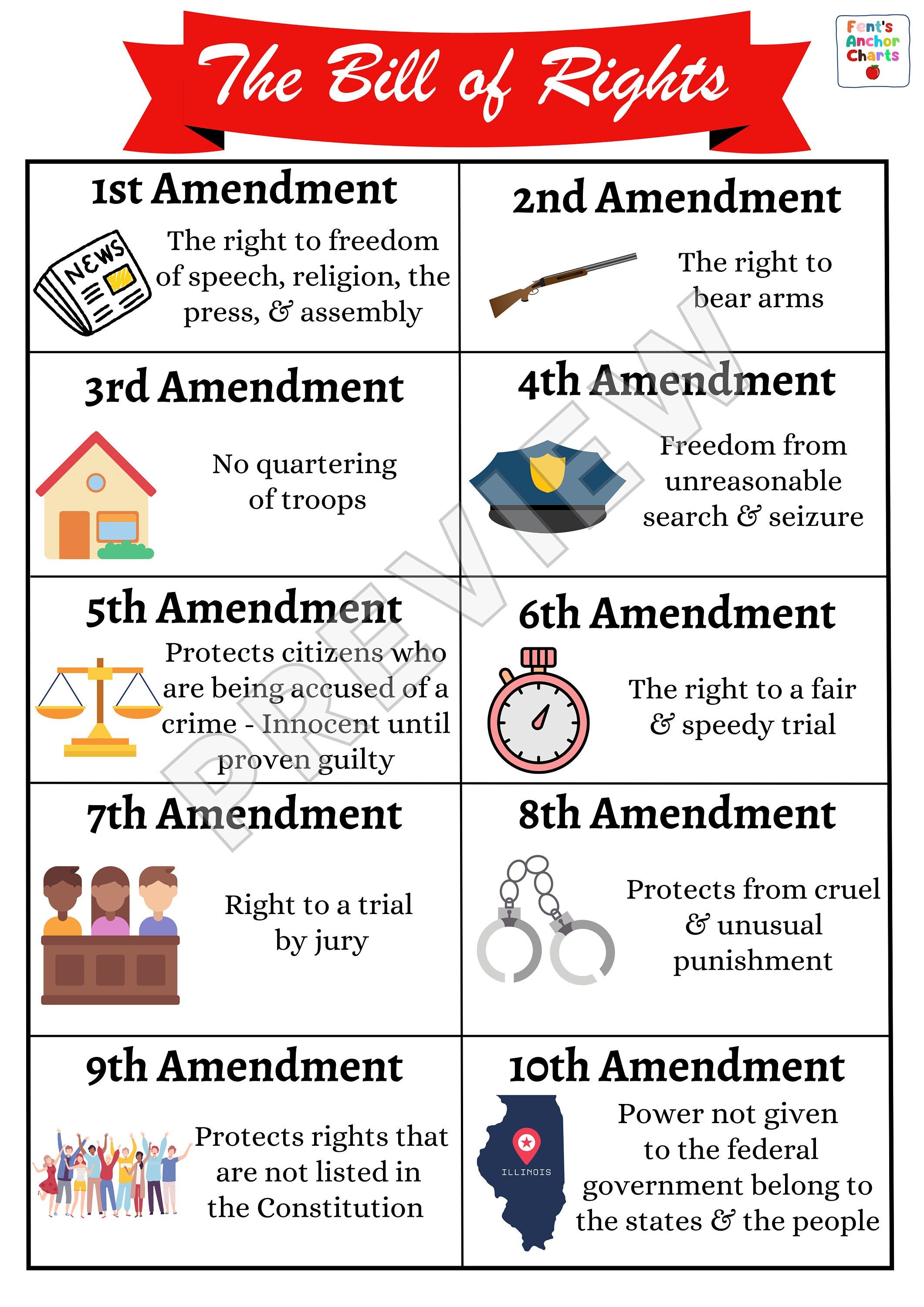
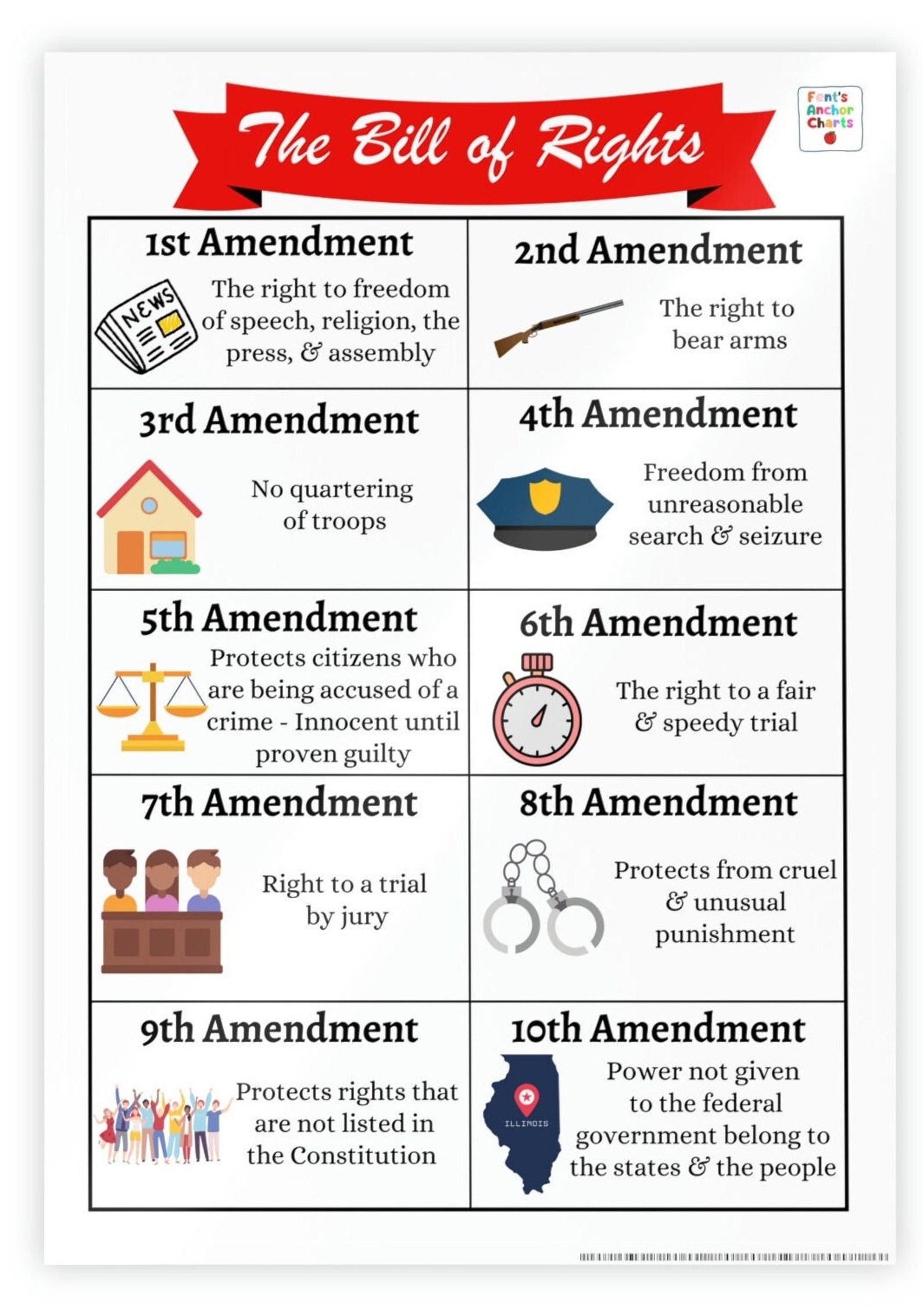
Bill of Rights Anchor Chart, 10 Amendments Anchor Chart, Social Studies Poster, U.S
Constitutions and Contracts Amending or Changing the Contract United States Government
First Amendment Anchor Chart, Bill of Rights Anchor Chart, Amendments Poster, United States
Periodic Table of Amendments Chart Teaching us history, Constitution activities, Civil war
Fourth Amendment
Bill of Rights Anchor Chart 10 Amendments Anchor Chart Etsy
The Guidance Put Forth By Byu Human Research Protection Program Borrows Extensively From The Best Practices Of A Variety Of Academic Institutions.
Amendments May Be Submitted At Anytime, However, The Change Must Not Be.
Related Post: